One-pot thermal decomposition of commercial organometallic salt to Fe2O3@C–N and MnO@C–N for lithium storage
Abstract
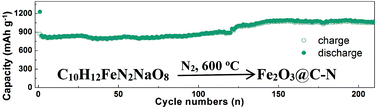
Iron oxide (Fe2O3) nanoparticles encapsulated in the N-doped carbon framework (Fe2O3@C–N) were synthesized via a one-step thermal decomposition reaction of commercial C10H12FeN2NaO8 (ethylenediaminetetraacetic acid monosodium ferric salt), which can serve as the source of Fe, O, C, and N. As an anode material for lithium storage, the Fe2O3@C–N sample exhibits a reversible capacity of 1072 mA h g−1 after 200 cycles at 0.2 A g−1 and 553 mA h g−1 after 500 cycles at 0.5 A g−1. Furthermore, the synthetic strategy can be simply extended to prepare other similar products, e.g. MnO@C–N and ZnO@C–N. The MnO@C–N anode also shows good cycling performances (915 mA h g−1 after 200 cycles at 0.2 A g−1 and 768 mA h g−1 after 500 cycles at 0.5 A g−1).


 Please wait while we load your content...
Please wait while we load your content...