Tuning of magnetic properties of the 2D CN-bridged NiII–NbIV framework by incorporation of guest cations of alkali and alkaline earth metals†
Abstract
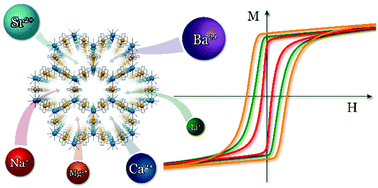
The reaction between [Ni(cyclam)]2+ (cyclam = 1,4,8,11-tetraazacyclotetradecane) and [Nb(CN)8]4− in concentrated water solutions of different s-block metal salts leads to the formation of 2-dimensional honeycomb-like coordination networks of the formula Mx[Ni(cyclam)]3[Nb(CN)8]2·nH2O (x = 2: M = Li+, Na+; x = 1: M = Mg2+, Ca2+, Sr2+, Ba2+). The CN-bridged Ni–Nb coordination layers are intersected by channels filled with crystallisation water molecules and guest mono- or di-valent metal cations, which compensate the negative charge of the framework. The structural details and crystal symmetry vary between the networks, depending on the arrangement of the water molecules and the intermolecular interactions enforced by the guest cations. All compounds show long range magnetic order arising from superexchange interactions between paramagnetic NiII (s = 1) and NbIV (s = 1/2) centres through CN-bridges within the layers and weaker inter-layer interactions mediated by H-bonds. The ordering temperature as well as the coercive field of the magnetic hysteresis can be tuned by the type of guest cation, with the highest values achieved for Mg2+ and the lowest for Na+.


 Please wait while we load your content...
Please wait while we load your content...