Selective cyclohexene oxidation to allylic compounds over a Cu-triazole framework via homolytic activation of hydrogen peroxide†
Abstract
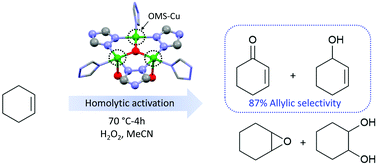
Utilization of metal–organic frameworks as heterogeneous catalysts is crucial owing to their abundant catalytic sites and well-defined porous structures. Highly robust [Cu3(trz)3(μ3-OH)(OH)2(H2O)4]·2H2O (trz = 1,2,4-triazole) was employed as a catalyst for liquid-phase cyclohexene oxidation with hydrogen peroxide (H2O2). Possessing the porous structure together with Lewis acid attributes from the triangular [Cu3(trz)3(μ3-OH)] center, selective oxidation of cyclohexene to allylic products gives a molar yield of 31% with 87% selectivity. According to the highly selective allylic production, the reaction over the present Cu-MOF plausibly occurs via homolytic activation of H2O2. This finding elucidates the unique features of the MOF for efficient catalysis of cyclohexene oxidation.


 Please wait while we load your content...
Please wait while we load your content...