The mechanism of the gold(i)-catalyzed Meyer–Schuster rearrangement of 1-phenyl-2-propyn-1-ol via 4-endo-dig cyclization†
Abstract
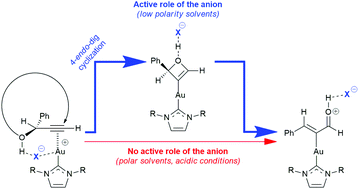
With the aim of rationalizing the experimental counterion- and solvent-dependent reactivity in the gold(I)-catalyzed Meyer–Schuster rearrangement of 1-phenyl-2-propyn-1-ol, a computational mechanistic study unraveled the unexpected formation of a gold-oxetene intermediate via commonly unfavorable 4-endo-dig cyclization triggered by the counterion in low polarity solvents.


 Please wait while we load your content...
Please wait while we load your content...