Designed pincer ligand supported Co(ii)-based catalysts for dehydrogenative activation of alcohols: Studies on N-alkylation of amines, α-alkylation of ketones and synthesis of quinolines†
Abstract
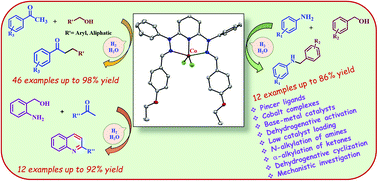
Base-metal catalysts Co1, Co2 and Co3 were synthesized from designed pincer ligands L1, L2 and L3 having NNN donor atoms respectively. Co1, Co2 and Co3 were characterized by IR, UV–Vis. and ESI-MS spectroscopic studies. Single crystal X-ray diffraction studies were investigated to authenticate the molecular structures of Co1 and Co3. Catalysts Co1, Co2 and Co3 were utilized to study the dehydrogenative activation of alcohols for N-alkylation of amines, α-alkylation of ketones and synthesis of quinolines. Under optimized reaction conditions, a broad range of substrates including alcohols, anilines and ketones were exploited. A series of control experiments for N-alkylation of amines, α-alkylation of ketones and synthesis of quinolines were examined to understand the reaction pathway. ESI-MS spectral studies were investigated to characterize cobalt-alkoxide and cobalt-hydride intermediates. Reduction of styrene by evolved hydrogen gas during the reaction was investigated to authenticate the dehydrogenative nature of the catalysts. Probable reaction pathways were proposed for N-alkylation of amines, α-alkylation of ketones and synthesis of quinolines on the basis of control experiments and detection of reaction intermediates.


 Please wait while we load your content...
Please wait while we load your content...