A family of rhodium(i) NHC chelates featuring O-containing tethers for catalytic tandem alkene isomerization–hydrosilylation†
Abstract
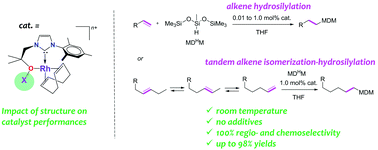
The rhodium complex Rh(HL)(COD)Cl, 1, L being a functionalized N-heterocyclic carbene (NHC) ligand with an oxygen-containing pendant arm, has been used as the entry point to synthesize a series of neutral and cationic Rh(I) O,C chelates. While the Rh–carbene interaction is similar in all these 16-electron complexes, structural analysis reveals that the strength of the Rh–O bond is greatly affected by the nature of the O-donor: R-O− > R-OH > R-OBF3. These subtle changes in the nature of the O-containing tether are found to be responsible for large differences in the alkene hydrosilylation catalytic activity of these compounds: the stronger the Rh–O interaction, the better the catalytic performances. The most active catalyst, [Rh(L)(COD)], 2, demonstrated good catalytic activity under mild reaction conditions for the hydrosilylation of a range of alkene substrates with the industrially relevant non-activated tertiary silane, 1,1,1,3,5,5,5-heptamethyltrisiloxane (MDHM). Furthermore, this complex is an effective catalyst for the selective remote functionalization of internal olefins at room temperature via tandem alkene isomerization–hydrosilylation.


 Please wait while we load your content...
Please wait while we load your content...