Stability, relaxometric and computational studies on Mn2+ complexes with ligands containing a cyclobutane scaffold†
Abstract
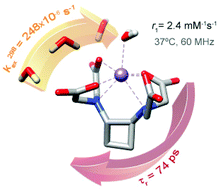
The stability constants of Mn2+ complexes with ligands containing a trans-1,2-cyclobutanediamine spacer functionalized with picolinate and/or carboxylate functions were determined using potentiometric titrations (25 °C, 0.1 M KCl). The stability constant of the complex with a hexadentate ligand containing four acetate groups (L14−, log KMnL = 10.26) is improved upon replacing one (L24−, log KMnL = 14.71) or two (L34−, log KMnL = 15.81) carboxylate groups with picolinates. The [Mn(L1)]2− complex contains a water molecule coordinated to the metal ion in aqueous solutions, as evidenced by 1H NMRD studies and 17O chemical shifts and transverse relaxation rates. The 1H relaxivities determined at 60 MHz (3.3 and 2.4 mM−1 s−1 at 25 and 37 °C, respectively) are comparable to those of monohydrated complexes such as [Mn(edta)]2−. The exchange rate of the inner-sphere water molecule (k298ex = 248 × 106 s−1) is slightly lower than that of the edta4− analogue. DFT calculations (M11/def2-TZVP) suggest that the water exchange reaction follows a dissociatively activated mechanism, providing activation parameters in reasonably good agreement with the experimental data. DFT calculations also show that the 17O hyperfine coupling constant A/ℏ is affected slightly by changes in the Mn–Owater distance and the orientation of the water molecule with respect to the Mn–O vector.


 Please wait while we load your content...
Please wait while we load your content...