Tuning the stereoelectronic factors of iron(ii)-2-aminophenolate complexes for the reaction with dioxygen: oxygenolytic C–C bond cleavage vs. oxidation of complex†
Abstract
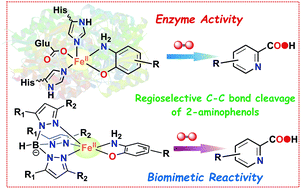
Oxidative C–C bond cleavage of 2-aminophenols mediated by transition metals and dioxygen is a topic of great interest. While the oxygenolytic C–C bond cleavage reaction relies on the inherent redox non-innocent property of 2-aminophenols, the metal complexes of 2-aminophenolates often undergo 1e−/2e− oxidation events (metal or ligand oxidation), instead of the direct addition of O2 for subsequent C–C bond cleavage. In this work, we report the isolation, characterization and dioxygen reactivity of a series of ternary iron(II)-2-aminophenolate complexes [(TpPh,Me)FeII(X)], where X = 2-amino-4-tert-butylphenolate (4-tBu-HAP) (1); X = 2-amino-4,6-di-tert-butylphenolate (4,6-di-tBu-HAP) (2); X = 2-amino-4-nitrophenolate (4-NO2-HAP)(3); and X = 2-anilino-4,6-di-tert-butylphenolate (NH-Ph-4,6-di-tBu-HAP) (4) supported by a facial tridentate nitrogen donor ligand (TpPh,Me = hydrotris(3-phenyl-5-methylpyrazol-1-yl)borate). Another facial N3 ligand (TpPh2 = hydrotris(3,5-diphenyl-pyrazol-1-yl)borate) has been used to isolate an iron(II)-2-anilino-4,6-di-tert-butylphenolate complex (5) for comparison. Both [(TpPh,Me)FeII(4-tBu-HAP)] (1) and [(TpPh,Me)FeII(4,6-di-tBu-HAP)] (2) undergo regioselective oxidative aromatic ring fission reaction of the coordinated 2-aminophenols to the corresponding 2-picolinic acids in the reaction with dioxygen. In contrast, complex [(TpPh,Me)FeII(4-NO2-HAP)] (3) displays metal based oxidation to form an iron(III)-2-amidophenolate complex. Complexes [(TpPh,Me)FeII(NH-Ph-4,6-di-tBu-HAP)] (4) and [(TpPh2)FeII(NH-Ph-4,6-di-tBu-HAP)] (5) react with dioxygen to undergo 2e− oxidation with the formation of the corresponding iron(III)-2-iminobenzosemiquinonato radical species implicating the importance of the –NH2 group in directing the C–C bond cleavage reactivity of 2-aminophenols. The systematic study presented in this work unravels the effect of the electronic and structural properties of the redox non-innocent 2-aminophenolate ring and the supporting ligand on the C–C bond cleavage reactivity vs. the metal/ligand oxidation of the complexes. The study further reveals that proper modulation of the stereoelectronic factors enables us to design a well synchronised proton transfer (PT) and dioxygen binding events for complexes 1 and 2 that mimic the structure and function of the nonheme enzyme 2-aminophenol-1,6-dioxygenase (APD).


 Please wait while we load your content...
Please wait while we load your content...