Syntheses, crystal structures, and biological evaluations of new dinuclear platinum(ii) complexes with 1,2,4-triazole derivatives as bridging ligands†
Abstract
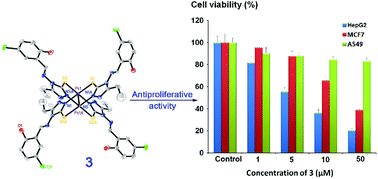
A series of new dinuclear platinum(II) complexes with the general formula [Pt2(μ-HL)4] (1–4), where H2L is 4-[(5-chloro-2-hydroxy-benzylidene)-amino]-3-R-1,2,4-triazole-5-thione: R = H (1), methyl (2), ethyl (3) and propyl (4), were synthesized and characterized. The X-ray crystal structures of 2, 3 and 4 reveal that the two platinum atoms form a paddlewheel core with four chelating triazole ligands as bridges, revealing a radically different structure than those of the traditional anticancer platinum(II) complexes. These complexes show higher in vitro antiproliferative activity against human liver hepatocellular carcinoma (HepG2) and human breast adenocarcinoma (MCF7) than human lung cancer (A549) and human normal hepatocyte (HL-7702) cell lines. In particular, 3 exhibits antiproliferative activity (IC50 = 5.5 μM) against HepG2 cells comparable to that of cisplatin. Different from the traditional anticancer platinum(II) complexes with high DNA affinity, 3 binds very weakly to DNA. Upon comparison, it exhibits potent inhibiting activity against protein tyrosine phosphatases 1B (PTP1B, IC50 = 16 μM) through possible binding to its active sites and its binding constant is 5.28 × 104 M−1. The results suggest that the antiproliferative mechanism of 3 against HepG2 cells may be different from that of cisplatin.


 Please wait while we load your content...
Please wait while we load your content...