COx-free hydrogen production via ammonia decomposition over mesoporous Co/Al2O3 catalysts with highly dispersed Co species synthesized by a facile method†
Abstract
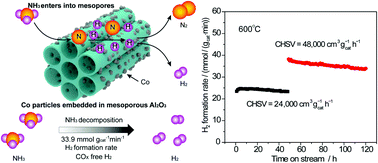
Transition metals have been considered as potential catalysts for ammonia decomposition to produce COx-free hydrogen for fuel cells. However, the facile synthesis of transition metal catalysts with small size active species, high porosity and good structural stability is still a challenge in catalytic NH3 decomposition. Herein, mesoporous Co/Al2O3 catalysts with various cobalt contents were synthesized by a facile modified sol–gel method. The catalyst 15CoAl with 15 at% cobalt content realizes the optimal catalytic NH3 decomposition performance. 92% NH3 conversion at 600 °C is achieved with a gaseous hourly space velocity (GHSV) of 24 000 cm3 gcat−1 h−1 and a hydrogen formation rate of 33.9 mmol gcat−1 min−1 at 600 °C is maintained after a 120 h long-duration stability test. Uniform small cobalt particles with high dispersion are well embedded into the skeleton of the mesoporous Al2O3 matrix. The aggregation of active cobalt species during the high temperature reaction can be effectively prevented by the mesoporous Al2O3 matrix due to the strong interaction between them, thus ensuring a good catalytic performance for ammonia decomposition.


 Please wait while we load your content...
Please wait while we load your content...