Direct synthesis of ring-fused quinolines and pyridines catalyzed by NNHY-ligated manganese complexes (Y = NR2 or SR)†
Abstract
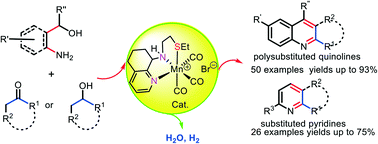
Four cationic manganese(I) complexes, [(fac-NNHN)Mn(CO)3]Br (Mn-1–Mn-3) and [(fac-NNHS)Mn(CO)3]Br (Mn-4) (where NNH is a 5,6,7,8-tetrahydro-8-quinolinamine moiety), have been synthesized and evaluated as catalysts for the direct synthesis of quinolines and pyridines by the reaction of a γ-amino alcohol with a ketone or secondary alcohol; NNHS-ligated Mn-4 proved the most effective of the four catalysts. The reactions proceeded well in the presence of catalyst loadings in the range 0.5–5.0 mol% and tolerated diverse functional groups such as alkyl, cycloalkyl, alkoxy, chloride and hetero-aryl. A mechanism involving acceptorless dehydrogenation coupling (ADC) has been proposed on the basis of DFT calculations and experimental evidence. Significantly, this manganese-based catalytic protocol provides a promising green and environmentally friendly route to a wide range of synthetically important substituted monocyclic, bicyclic as well as tricyclic N-heterocycles (including 50 quinoline and 26 pyridine examples) with isolated yields of up to 93%.


 Please wait while we load your content...
Please wait while we load your content...