The first one-pot metathesis–hydroformylation procedure: a straight synthesis of 2-arylpropanals from renewable 1-propenylbenzenes†
Abstract
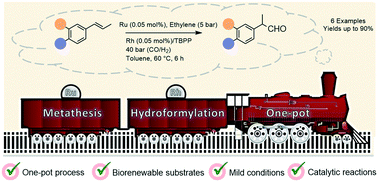
Hydroformylation is a consolidated synthetic tool in the chemical industry, both in commodity and in the fine chemicals industry. Olefin metathesis has been largely employed in the petrochemical sector, and, more recently, in the synthesis of specialty chemicals. Although these reactions may be involved in the same synthetic route for various industrial chemicals, to the best of our knowledge, they have never been combined in a one-pot procedure. As a proof of concept, we have demonstrated in the present work that the ruthenium-catalyzed ethenolysis of renewable 1-propenylbenzenes followed by the rhodium-catalyzed hydroformylation of functionalized styrenes formed in the first step could be done in one pot. The integration of these reactions was not straightforward once the catalyst of the first step interfered with the catalyst of the second step. Under optimized conditions, it was possible to synthesize 2-arylpropanals, a class of compounds valuable as synthetic intermediates to access non-steroidal anti-inflammatory drugs, in overall yields of 85–90%, at low catalyst loadings.


 Please wait while we load your content...
Please wait while we load your content...