Origins of ligand-controlled diastereoselectivity in dirhodium-catalysed direct amination of aliphatic C(sp3)–H bonds†
Abstract
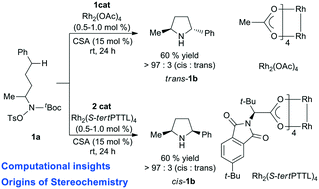
The mechanism and origins of ligand-controlled stereoselectivity of dirhodium (Rh2L4, L = OAc, S-tertPTTL)-catalysed intramolecular aliphatic C(sp3)–H aminations have been explored using DFT calculations. Both catalytic cycles occur via the formation of Rh2 = nitrene radical species, intramolecular H-abstraction and radical rebound. The origins of diastereoselectivity are revealed via distortion/interaction and noncovalent interaction analysis.


 Please wait while we load your content...
Please wait while we load your content...