Shape selectivity effects in the hydroconversion of perhydrophenanthrene over bifunctional catalysts†
Abstract
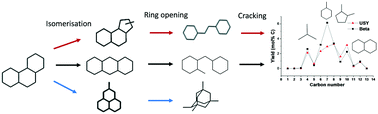
Hydroconversion of perhydrophenanthrene was performed over Pt/Beta and Pt/ASA bifunctional catalysts and compared to results obtained on the Pt/USY zeolite catalyst, under the same operating conditions. Perhydrophenanthrene resulted from the hydrogenation of the parent aromatic, phenanthrene, on a Pt/alumina pre-catalyst. All three bifunctional catalysts followed the general reaction pathway observed previously, i.e. isomerization of perhydrophenanthrene by ring-shift and ring-contraction, followed either by the formation of alkyladamantanes or by ring-opening. The ring opening products cracked to smaller naphthenes. Yet, the intermediates of this general reaction network differed very clearly from one catalyst to another. These shape selectivity effects could be explained by GCMC simulations of the adsorption selectivities of different intermediates. Bulky intermediates were preferentially adsorbed on USY zeolites, whereas Beta zeolites adsorbed preferentially linear structures. The product distribution on Beta zeolites was explained through the formation of a central ring-opening intermediate which cracked rapidly to C7 naphthenes. USY-based catalysts were the most active among the solids tested, while Beta zeolites were slightly more selective to generate cracked products.


 Please wait while we load your content...
Please wait while we load your content...