The solvent determines the product in the hydrogenation of aromatic ketones using unligated RhCl3 as catalyst precursor†
Abstract
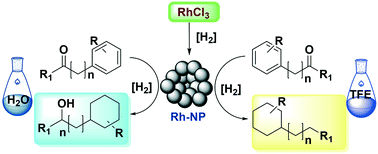
Alkyl cyclohexanes were synthesized in high selectivity via a combined hydrogenation/hydrodeoxygenation of aromatic ketones using ligand-free RhCl3 as pre-catalyst in trifluoroethanol as solvent. The true catalyst consists of rhodium nanoparticles (Rh NPs), generated in situ during the reaction. A range of conjugated as well as non-conjugated aromatic ketones were directly hydrodeoxygenated to the corresponding saturated cyclohexane derivatives at relatively mild conditions. The solvent was found to be the determining factor to switch the selectivity of the ketone hydrogenation. Cyclohexyl alkyl-alcohols were the products using water as a solvent.


 Please wait while we load your content...
Please wait while we load your content...