FeVO4-supported Mn–Ce oxides for the low-temperature selective catalytic reduction of NOx by NH3†
Abstract
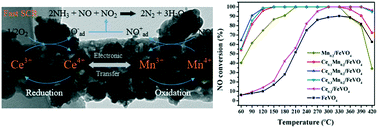
Iron vanadate (FeVO4) nanorods are used as a carrier to support manganese (Mn) and cerium (Ce) oxides for the selective catalytic reduction (SCR) of nitrogen oxides (NOx) with NH3 for the first time. Among these developed Ce–Mn/FeVO4 catalysts with different molar ratios of Ce/Mn, the Ce0.2Mn0.2/FeVO4 catalyst exhibits the best de-NOx performance and N2 selectivity, which are higher than 90% in a wide temperature window of 90–420 °C. Through a series of characterization techniques, it is found that the synergistic effect between Ce and Mn enhances the reduction ability and the number of acid sites on the catalyst, which facilitates the adsorption and conversion of flue gas. The introduction of an appropriate ratio of Ce/Mn increases the concentration of Mn4+ and chemisorbed oxygen (OS) on the catalyst, leading to a “fast SCR reaction” with oxidizing NO to NO2, which significantly improves the low-temperature de-NOx efficiency. In addition, the interaction between the active components (Ce/Mn) and the support (FeVO4) increases the de-NOx performance of Ce0.2Mn0.2/FeVO4 at high temperatures. In the meantime, the Mn4+ + Ce3+ ↔ Mn3+ + Ce4+ reduction electron pair formed between Ce and Mn promotes the transport of electrons, which is also beneficial for the SCR reaction at low temperature. The in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) reveals that the SCR reaction over Ce0.2Mn0.2/FeVO4 catalyst follows both Eley–Rideal (E–R) and Langmuir–Hinshelwood (L–H) reaction mechanisms.


 Please wait while we load your content...
Please wait while we load your content...