Structure of the fungal hydroxylase, CYP505A30, and rational transfer of mutation data from CYP102A1 to alter regioselectivity†
Abstract
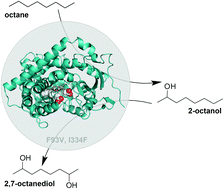
CYP505A30 is a fungal, self-sufficient cytochrome P450 monooxygenase that can selectively oxyfunctionalise n-alkanes, fatty alcohols, and fatty acids. From alkanes, it produces a mixture of non-vicinal diols by two sequential hydroxylation reactions. Here we report the structure of the haem domain of CYP505A30, the first structure for a member of the CYP505 family, with dodecanoic acid bound within the active site. Overall, a high structural similarity to the related bacterial CYP102A1 was observed, despite low sequence identity (<40%). Comparison of the active sites, however, showed a high degree of conservation with only two amino acid differences close to the haem. Stabilisation of the fatty acid substrate in CYP505A30 also occurs, as in CYP102A1, via an arginine residue. However, compared to R47, which is situated in the β1 region of CYP102A1, R358 is located in the β3 region of CYP505A30. We furthermore created mutants to test if it is possible to rationally transfer the knowledge on active site mutations in CYP102A1 to change the regioselectivity of CYP505A30. The introduction of F93V, I334F mutations resulted in increased ω-1 (C2) regioselectivity, similar to CYP102A1 87-328, of more than 80% for n-octane and 90% for n-decane. Changing residues to resemble the CYP102A1 wildtype increased the regioselectivity towards ω-2 (C3) to over 60% for both substrates. The knowledge gained from this study unlocks a more selective production of symmetrical non-vicinal diols from n-alkanes.


 Please wait while we load your content...
Please wait while we load your content...