Nickel-catalysed fluoromethylation reactions
Abstract
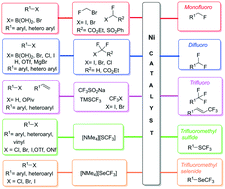
Owing to the advantages offered by fluorine in the pharmaceutical and agrochemical industry, methods to incorporate fluorine have gained immense attention in the last two decades. Recently, several sustainable catalysts based on first row transition metals have been employed for fluoromethylation reactions. Amongst them, nickel catalysts due to their abundance, less toxicity and high catalytic activity have been increasingly used for the introduction of a variety of fluorinated functionalities like mono-, di- and tri-fluoromethyl groups including trifluoromethyl selenides and sulfides. This is the first review that exclusively covers the important strategies and mechanistic analyses in nickel-catalysed mono-, di- and tri-fluoromethylation, trifluoromethylthiolation and trifluoromethylselenolation covering the literature up to 2021.


 Please wait while we load your content...
Please wait while we load your content...