Ethanol steam reforming on Rh: microkinetic analyses on the complex reaction network†
Abstract
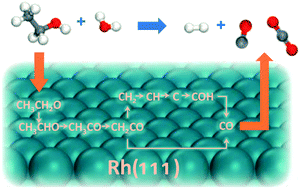
Ethanol steam reforming is one of the most widely used processes for hydrogen production, but the mechanism of the whole reaction pathway from ethanol to CO and CO2 has not been studied, possibly due to the complex reaction network of this reaction. In this work, we used a method developed recently by our group to determine the preferred reaction pathways under certain conditions from a large network, based on density functional theory (DFT) calculations and microkinetic modeling, and reaction networks with 108 and 110 elementary steps were generated for CO and CO2 formation, respectively. After the pruning of these networks, we found that at 923–1073 K, the mechanisms of ethanol to CO or CO2 should be CH3CH2OH → CH3CH2O* → CH3CHO* → CH3CO* → CH2CO* → 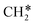 + CO* → CH* + CO* → C* + CO* → COH* + CO* → (2CO* → 2CO or 2CO* + 2O* → 2CO2). Further microkinetic analyses combining the formation of CO and CO2 showed that CH3CH2OH → CH3CH2O + H is the rate determining step of the ethanol steam reforming, and all the coverages of surface species are lower than 0.1 monolayer on Rh(111) at 923–1073 K. We also found that the increase of temperature is beneficial to the production rate of both CO and CO2, while only the rate of CO2 increases with the increase of the H2O molar ratio in reactants.
+ CO* → CH* + CO* → C* + CO* → COH* + CO* → (2CO* → 2CO or 2CO* + 2O* → 2CO2). Further microkinetic analyses combining the formation of CO and CO2 showed that CH3CH2OH → CH3CH2O + H is the rate determining step of the ethanol steam reforming, and all the coverages of surface species are lower than 0.1 monolayer on Rh(111) at 923–1073 K. We also found that the increase of temperature is beneficial to the production rate of both CO and CO2, while only the rate of CO2 increases with the increase of the H2O molar ratio in reactants.


 Please wait while we load your content...
Please wait while we load your content...