Low-temperature Fe–MnO2 nanotube catalysts for the selective catalytic reduction of NOx with NH3†
Abstract
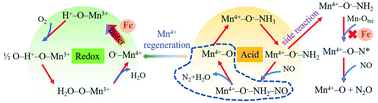
Exhaust gas from the sintering process in the iron and steel industry contains NOx and SO2 at low temperature. Therefore, the corresponding denitration catalyst should have favorable low-temperature activity and SO2 resistance. In this study, an Fe-doped MnO2 nanotube catalyst with the (211) crystal face synthesized using a one-step hydrothermal synthesis method demonstrated favorable pore structure and many weak acidic sites, which improved its low-temperature activity (>90% at 125–225 °C), N2 selectivity (>90%), and SO2 resistance. The NO conversion rate reached 94% when the Fe0.5%MnO2 catalyst was used in the presence of SO2. Moreover, the Fe in the Fe0.5%MnO2 catalyst improved the reducibility of the surface Mn sites and reduced the oxidizing properties of bridging oxygen, resulting in easier conversion of Mn4+ to Mn3+ and affecting the regeneration of oxygen vacancies in the selective catalytic reduction of NOx with NH3 process. In situ diffuse reflectance infrared spectroscopy showed that the reduction of NOx in the MnO2 nanotubes and Fe0.5%MnO2 sample involved the Eley–Rideal mechanism, whereas the NH3-SCR process of the Fe5%MnO2 sample involved both Langmuir–Hinshelwood and Eley–Rideal mechanisms.


 Please wait while we load your content...
Please wait while we load your content...