Revisiting trends in the exchange current for hydrogen evolution†
Abstract
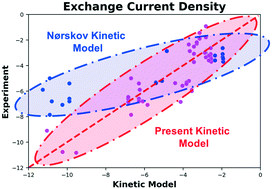
Nørskov and collaborators proposed a simple kinetic model to explain the volcano relation for the hydrogen evolution reaction on transition metal surfaces such that j0 = k0f(ΔGH) where j0 is the exchange current density, f(ΔGH) is a function of the hydrogen adsorption free energy ΔGH as computed from density functional theory, and k0 is a universal rate constant. Herein, focusing on the hydrogen evolution reaction in acidic medium, we revisit the original experimental data and find that the fidelity of this kinetic model can be significantly improved by invoking metal-dependence on k0 such that the logarithm of k0 linearly depends on the absolute value of ΔGH. We further confirm this relationship using additional experimental data points obtained from a critical review of the available literature. Our analyses show that the new model decreases the discrepancy between calculated and experimental exchange current density values by up to four orders of magnitude. Furthermore, we show the model can be further improved using machine learning and statistical inference methods that integrate additional material properties.


 Please wait while we load your content...
Please wait while we load your content...