Hydroxy acid-functionalized ionic liquids as green alternatives for the efficient catalytic conversion of epoxides to cyclic carbonates under solvent and co-catalyst-free conditions†
Abstract
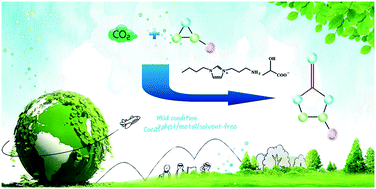
An innovative, efficient, experimentally simple and environmentally friendly synthesis route of cyclic carbonates from carbon dioxide and epoxide applying novel hydroxy acid-functionalized ionic liquids as efficient catalysts under solvent and co-catalyst-free conditions has been developed. These hydroxy acid-functionalized ionic liquids exhibit outstanding catalytic activity for the conversion of CO2 and various epoxides to cyclic carbonates. The effects of reaction parameters (amount of catalyst, CO2 pressure, reaction time and temperature) on the catalytic activity for chloropropene carbonate synthesis from epichlorohydrin and CO2 are examined and optimized. The catalysts could be reused for at least seven times without any noticeable loss of selectivity and activity. The activation energy was determined to be 66.9 kJ mol−1. Finally, a possible reaction mechanism is proposed.


 Please wait while we load your content...
Please wait while we load your content...