Nb-Doped nickel nitride-derived catalysts for electrochemical water splitting†
Abstract
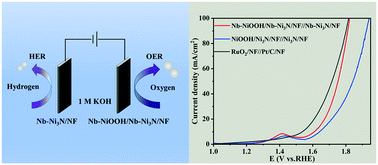
The design and synthesis of noble metal-free bifunctional electrocatalysts with high performance are of critical importance for the electrochemical splitting of water. This study proposes that Nb-doped nickel nitride nanosheets supported on Ni foam (Nb–Ni3N/NF) as novel and effective catalysts demonstrate enhanced electrocatalytic activity for the hydrogen evolution reaction (HER) and oxygen evolution reduction (OER) compared to the pristine Ni3N samples. DFT calculations reveal that the Nb doping optimizes the Gibbs free energy of hydrogen adsorption (ΔGH*) close to zero and lowers the d-band center of Ni, facilitating the HER process. Meanwhile, the improved OER performance is due to the Nb dopant enhancing the catalytic activity of NiOOH, which in situ formed on the Ni3N surface during anodic oxidation. An alkaline electrolyzer using Nb–Ni3N/NF as electrodes can realize a current density of 10 mA cm−2 at a cell voltage of 1.61 V, even close to that of the combination of Pt and RuO2.


 Please wait while we load your content...
Please wait while we load your content...