Selective electroreduction of CO2 to ethanol over a highly stable catalyst derived from polyaniline/CuBi2O4†
Abstract
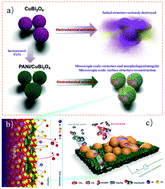
Electroreduction of CO2 to ethanol with high stability and selectivity is vital for resourceful CO2 utilization. The catalyst derived from PANi/CuBi2O4via an electrochemical activation process shows high faraday efficiency (FE) for the electroreduction of CO2 to ethanol (∼64.15%). Repeated redox reaction generated the evolutionary surface of PANi/CuBi2O4 with Cu0+ and Bi0+ crossed. Owing to the high priority of the adsorbed O-terminal on the introduced Bi, which is located on the Cu surface, the CO2RR pathway for ethylene is suppressed after C–C coupling, making PANi/CuBi2O4 more likely to produce ethanol. With our thorough investigation of the PANi/CuBi2O4 composite system, the agglomerate behaviour of the catalyst in the activation process can be effectively suppressed leading to the high stability and long lifetime of the catalyst. Our findings highlight the importance of local surface evolution by electrochemical pretreatment while retaining the excellent property by stabilizing the main body of the catalyst.


 Please wait while we load your content...
Please wait while we load your content...