Structure-guided evolution of a ketoreductase for efficient and stereoselective bioreduction of bulky α-amino β-keto esters†
Abstract
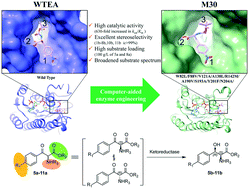
Ketoreductases have shown considerable potential as biocatalysts in the asymmetric synthesis of chiral alcohols. However, compared to the widely studied ketoreductases for chiral alcohols, limited knowledge is available about ketoreductases for efficient dynamic reductive kinetic resolution (DYRKR) of bulky α-amino β-keto esters. In this study, structure-guided rational direct evolution was applied to a ketoreductase (WTEA) from Exiguobacterium sp. F42 for the asymmetric reduction of bulky α-amino β-keto esters. A number of mutants were then obtained with remarkably improved activity toward various bulky α-amino β-keto esters with excellent stereoselectivity by performing structure-guided rational design. In particular, mutant M30 (W82L/F88V/V121A/A138L/R142M/A190V/S193A/Y201F/N204A) exhibited excellent stereoselectivity (>99% dr, >99% de) and high conversion (>99%) for six α-amino β-keto esters. Furthermore, novel and practical chemoenzymatic routes were developed for the synthesis of chloramphenicol and florfenicol, which featured the application of enzymatic DYRKR to establish the two stereocenters of amino alcohols 4-NO2-substituted (2S, 3R)-5b and 4-SO2Me-substituted (2S, 3R)-8b with > 99% dr, > 99% de and >99% conversion from 100 g L−1 α-amino β-keto esters 5a and 8a (highest substrate loading reported), respectively. Crystal structure and molecular dynamics studies revealed the potential molecular basis for activity improvement and the stereoselectivity control mechanism at the atomic level. These results provide important insights into the evolution of ketoreductases for the asymmetric synthesis of chiral vicinal amino alcohols and establish a solid foundation for further large-scale industrial applications in the future.


 Please wait while we load your content...
Please wait while we load your content...