Surface structure sensitivity of hydrodeoxygenation of biomass-derived organic acids over palladium catalysts: a microkinetic modeling approach†
Abstract
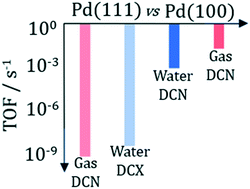
Microkinetic models based on parameters obtained from density functional theory and transition state theory have been developed for the hydrodeoxygenation (HDO) of propanoic acid, a model lignocellulosic biomass-derived organic acid, over the flat Pd(100) and Pd(111) surfaces in both vapor and liquid phase reaction conditions. The more open Pd(100) surface was found to be 3–7 orders of magnitude more active than the Pd(111) surface in all reaction environments, indicating that the (111) surface is not catalytically active for the HDO of propanoic acid. Over Pd(100) and in vapor phase, liquid water, and liquid 1,4-dioxane, propanoic acid hydrodeoxygenation follows a decarbonylation (DCN) mechanism that is facilitated by initial α- and β-carbon dehydrogenation steps, prior to the rate controlling C–OH and (partially rate controlling) C–CO bond dissociations. Only over Pd(111) and aqueous reaction environments is the decarboxylation (DCX) preferred over the DCN with the C–CO2 step being rate controlling.


 Please wait while we load your content...
Please wait while we load your content...