Reshaping the active pocket of esterase Est816 for resolution of economically important racemates†
Abstract
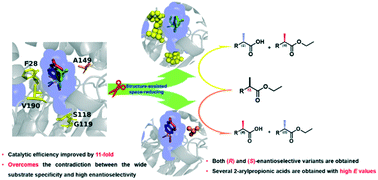
Bacterial esterases are potential biocatalysts for the production of optically pure compounds. However, the substrate promiscuity and chiral selectivity of esterases usually have a negative correlation, which limits their commercial value. Herein, an efficient and versatile esterase (Est816) was identified as a promising catalyst for the hydrolysis of a wide range of economically important substrates with low enantioselectivity. We rationally designed several variants with up to 11-fold increased catalytic efficiency towards ethyl 2-arylpropionates, mostly retaining the initial substrate scope and enantioselectivity. These variants provided a dramatic increase in efficiency for biocatalytic applications. Based on the best variant Est816-M1, several variants with higher or inverted enantioselectivity were designed through careful analysis of the structural information and molecular docking. Two stereoselectively complementary mutants, Est816-M3 and Est816-M4, successfully overcame and even reversed the low enantioselectivity, and several 2-arylpropionic acid derivatives with high E values were obtained. Our results offer potential industrial biocatalysts for the preparation of structurally diverse chiral carboxylic acids and further lay the foundation for improving the catalytic efficiency and enantioselectivity of esterases.


 Please wait while we load your content...
Please wait while we load your content...