Stereo-selective synthesis of non-canonical γ-hydroxy-α-amino acids by enzymatic carbon–carbon bond formation†
Abstract
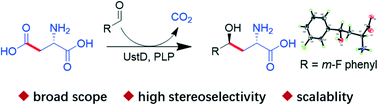
Carbon–carbon (C–C) bond formation is the fundamental reaction type in organic synthesis. Biocatalytic methods for C–C bond formation have been limited to a few types of enzymes. In this report, we demonstrated the capability of a PLP-dependent enzyme ApUstD performing both C–C bond activation and asymmetric C–C bond formation, which resulted in non-canonical γ-hydroxy-α-amino acids. The reaction showed high efficiency (conversion up to 98%), stereo-selectivity (ratio up to >97 : 3), a broad substrate scope (25 isolated examples) and significant simplicity. Our results have extended the biocatalytic function of PLP-dependent enzymes for asymmetric C–C formation.


 Please wait while we load your content...
Please wait while we load your content...