KF-Promoted copper-catalyzed highly efficient and selective oxidation of methane and other alkanes with a dramatic additive effect†
Abstract
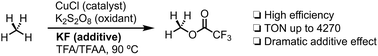
Highly efficient oxidation of methane to methyl trifluoroacetate mediated by CuCl/KF/K2S2O8 in trifluoroacetic acid (TFA) and trifluoroacetic anhydride (TFAA) is described. The additive effect has been systematically evaluated and potassium fluoride (KF) was identified as the most effective promoter among the salts screened. KF is conjectured to exhibit the salt effect to promote the [SO4˙]− radical to escape the solvent cage based on control experiments. Cyclohexane and adamantane could also be efficiently oxidized into benzene or corresponding trifluoroacetates.


 Please wait while we load your content...
Please wait while we load your content...