Tandem synthesis of tetrahydroquinolines and identification of the reaction network by operando NMR†
Abstract
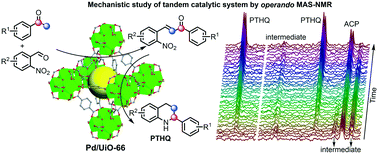
The study of the reaction mechanism and complex network for heterogeneously catalyzed tandem reactions is challenging but can guide reaction design and optimization. Here, we describe a case study using bifunctional metal–organic framework supported Pd nanoparticles (Pd/UiO-66(HCl)) for the one-pot tandem synthesis of substituted tetrahydroquinolines via the Claisen–Schmidt condensation and reductive intramolecular cyclization. The directly observed evolution of intermediates and products, including reactive species containing hydroxylamine group and unstable intermediate 2-phenyl-3,4-dihydroquinoline, was enabled by operando magic angle spinning nuclear magnetic resonance studies under 50 bar H2. The reaction network of the tandem reaction is deduced based on reaction kinetic information obtained from the operando study. The optimized procedure was applied to various acetophenone and nitrobenzaldehyde derivatives carrying different functional groups, and eight valuable substituted tetrahydroquinolines were obtained in moderate to good yields. This work provides a molecular-level understanding of the catalytic system and brings up new opportunities for efficient and sustainable synthesis of medicinally relevant building blocks.


 Please wait while we load your content...
Please wait while we load your content...