Ultralow loading of ruthenium nanoparticles on nitrogen-doped porous carbon enables ultrahigh mass activity for the hydrogen evolution reaction in alkaline media†
Abstract
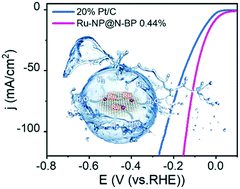
Ruthenium (Ru) has been proved to be a viable alternative to platinum (Pt) for the hydrogen evolution reaction (HER). However, it is still highly desirable to further raise the efficiency and diminish the mass-loading of Ru-based catalysts owing to the same scarcity problem as Pt. Herein, carbon-supported ultrafine Ru nanoparticles (ca. 1.4–2.0 nm) with the Ru loadings ranging from 0.25 to 5 wt% (denoted as Ru-NP@N-BP 0.25–5%) have been fabricated through initial aggregation of RuTTPP on the surface of carbon black BP 2000 (BP) followed by high temperature treatment. Employment of the RuTTPP molecular aggregates simultaneously as the metal nanoparticle and N resource has been found to allow the control over the location of the N atoms doped into the carbon matrix near the Ru nanoparticles formed due to the strong coordination interactions between Ru ions and N atoms originally existing in the molecular precursor on the basis of transmission electron microscopy and X-ray photoelectron spectroscopy. This results in effective interactions between the N dopants and Ru nanoparticles, stabilizing the Ru nanoparticles and enhancing the catalytic activity of Ru-NP@N-BPs. In particular, the as-prepared Ru-NP@N-BP 0.44% with ultralow ruthenium loading exhibits superior HER activity to Pt/C in 1.0 M KOH with a small overpotential of ca. 10.5 mV at 10 mA cm−2 and a low Tafel slope of 28.1 mV dec−1 as well as long-term durability. More importantly, also owing to the ultralow Ru loading, Ru-NP@N-BP 0.44% achieves a record-high mass activity of 24 A mgRu−1 at an overpotential of 50 mV in alkaline media among ever reported Ru-based HER catalysts. The excellent performance was further rationalized by the strong adsorption and fast dissociation of H2O together with the spontaneous desorption of *OH over the N-doped carbon-supported Ru nanoparticles in alkaline solution on the basis of the density functional theory calculations.


 Please wait while we load your content...
Please wait while we load your content...