Heterolytic alkene oxidation with H2O2 catalyzed by Nb-substituted Lindqvist tungstates immobilized on carbon nanotubes†
Abstract
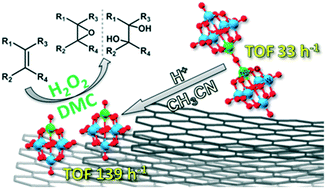
Nb-Monosubstituted polyoxometalates (Nb-POM) of the Lindqvist structure, monomers [Nb(L)W5O19]n− (Nb(L)W5, L = O, OCH3, (O)2; n = 3 or 2) and a dimer [(NbW5O18)2O]4−, were successfully immobilized on N-free and N-doped carbon nanotubes (CNTs and N-CNTs) and characterized by elemental analysis, N2 adsorption, XPS, FTIR spectroscopy, HR-TEM, and HAADF-STEM. The supported catalysts revealed superior activity and selectivity for the heterolytic oxidation of alkenes with aqueous hydrogen peroxide as a green oxidant to produce epoxides and diols as the principal oxidation products. Both CNTs and N-CNTs stabilize the monomeric form [Nb(OH)W5O18]2−, preventing its deactivation through dimerization and thereby leading to a great increase in the catalyst activity and productivity relative to homogeneous Nb(L)W5. Although N-doping ensures a more homogeneous dispersion of Nb-POMs on the surface, it enhances unproductive decomposition of the oxidant, leading to the enhancement of homolytic oxidation routes. The elaborated Nb-POM/CNT catalysts reveal the truly heterogeneous nature of the catalysis, and can be easily recovered by filtration, regenerated, and reused several times without a significant loss of the catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...