Engineering the large pocket of an (S)-selective transaminase for asymmetric synthesis of (S)-1-amino-1-phenylpropane†
Abstract
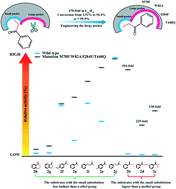
Amine transaminases offer an environmentally benign chiral amine asymmetric synthesis route. However, their catalytic efficiency towards bulky chiral amine asymmetric synthesis is limited by the natural geometric structure of the small pocket, representing a great challenge for industrial applications. Here, we rationally engineered the large binding pocket of an (S)-selective ω-transaminase BPTA from Paraburkholderia phymatum to relieve the inherent restriction caused by the small pocket and efficiently transform the prochiral aryl alkyl ketone 1-propiophenone with a small substituent larger than the methyl group. Based on combined molecular docking and dynamic simulation analyses, we identified a non-classical substrate conformation, located in the active site with steric hindrance and undesired interactions, to be responsible for the low catalytic efficiency. By relieving the steric barrier with W82A, we improved the specific activity by 14-times compared to WT. A π–π stacking interaction was then introduced by M78F and I284F to strengthen the binding affinity with a large binding pocket to balance the undesired interactions generated by F44. T440Q further enhanced the substrate affinity by providing a more hydrophobic and flexible environment close to the active site entry. Finally, we constructed a quadruple variant M78F/W82A/I284F/T440Q to generate the most productive substrate conformation. The 1-propiophenone catalytic efficiency of the mutant was enhanced by more than 470-times in terms of kcat/KM, and the conversion increased from 1.3 to 94.4% compared with that of WT, without any stereoselectivity loss (ee > 99.9%). Meanwhile, the obtained mutant also showed significant activity improvements towards various aryl alkyl ketones with a small substituent larger than the methyl group ranging between 104- and 230-fold, demonstrating great potential for the efficient synthesis of enantiopure aryl alkyl amines with steric hindrance in the small binding pocket.


 Please wait while we load your content...
Please wait while we load your content...