In situ sulfation of Cu/TiO2 catalysts for catalytic combustion of dichloromethane†
Abstract
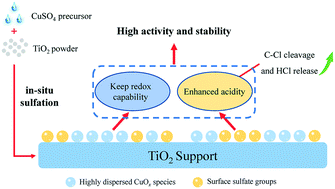
Herein, a facile in situ sulfation strategy was employed for modifying Cu/TiO2 catalysts using CuSO4 as the precursor to enhance the performance of dichloromethane (DCM) catalytic combustion. The experimental results revealed that the modified samples exhibited superior DCM conversion and COx yield compared to the pristine Cu/TiO2 catalyst. Among these, the 1CuSO4/TiO2 sample showed the best activity with near 90% conversion during 50 h of reaction at 275 °C. Characterization results showed that the in situ sulfation modification could considerably improve the catalyst acidity, benefiting the dissociative adsorption of DCM with C–Cl rupture. Notably, the surface sulfate groups provided abundant Brønsted acidic sites that release surface Cl species in the form of HCl at relatively low temperatures, preventing the surface Cu species from chloride poisoning. With excess CuSO4 addition, the intermediates derived from DCM dechlorination could not be decomposed in time due to the decreased reducibility, leading to their deactivation due to coke deposition. The superior performance of 1CuSO4/TiO2 is attributed to the balance achieved between acidity and redox capacity for DCM catalytic decomposition. Moreover, it was found that CuSO4/TiO2 could partially replace noble-metal Ru/TiO2 catalysts and achieve comparable performances. The findings of this study will aid the development of CVOCs catalytic combustion catalysts with good activity and stability.


 Please wait while we load your content...
Please wait while we load your content...