KnowVolution of prodigiosin ligase PigC towards condensation of short-chain prodiginines†
Abstract
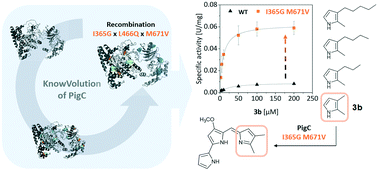
Prodigiosin ligase PigC catalyses the final condensation step in the prodigiosin biosynthesis of Serratia marcescens, in which two pyrrolic precursor molecules are fused linearly to form tripyrrolic red prodiginines. Prodiginine compounds attracted interest as pharmaceutical agents lately since their structural elucidation in the 1960s due to their multiple bioactivities (e.g., antibiotic, nematicidal or anticancer effects). As a key enzyme in the prodigiosin biosynthesis pathway, PigC presents a bottleneck for efficient broad-range prodiginine production because of its limited substrate acceptance. In particular, biosynthesis of short-chain prodiginines is of high synthetic interest due to their effective anticancer properties. In this study, PigC was successfully engineered to efficiently accept short-chain pyrroles following the KnowVolution strategy, which provided variants with up to 10.7-fold increased kcat (7.5 ± 0.4 min−1 compared to 0.7 ± 0.1 min−1 for the PigC wild type), and a nearly 40-fold enhanced catalytic efficiency (kcat/KM = 23.9 ± 3.6 mM−1 s−1 compared to 0.6 ± 0.1 mM−1 s−1 for the PigC wild type) towards short-chain pyrroles. The final and most active PigC variant S1 carried two amino acid substitutions (I365G/M671V) in the substrate-binding domain close to a substrate tunnel, and showed a pronounced preference for small pyrroles. In summary, the directed evolution campaign on PigC provided engineered variants to open a biosynthetic route for short-chain prodiginines.


 Please wait while we load your content...
Please wait while we load your content...