Biocatalysed synthesis of chiral amines: continuous colorimetric assays for mining amine-transaminases†
Abstract
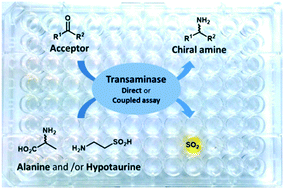
In the course of our research aimed at the design of new biocatalytic processes for the enantioselective synthesis of chiral amines, we have developed new continuous assays for the screening of amine-transaminase collections. These assays are based on the use of hypotaurine as an irreversible amine donor. This β-aminosulfinic acid is converted upon transamination into 2-oxoethylsulfinic acid, which instantaneously decomposes into acetaldehyde and sulfite ions that can be easily detected by spectrophotometry using Ellman's reagent. Two complementary assays were developed based on this titration method. Firstly, a direct assay allowed detection of various transaminases able to use hypotaurine as an amino donor. In a second coupled assay, L-alanine is used as a generic donor substrate of amine-transaminases and is regenerated using an auxiliary hypotaurine-transaminase. The powerful and complementary nature of both assays was demonstrated through the screening of a collection of 549 amine-transaminases from biodiversity, thus allowing the discovery of a variety of valuable new biocatalysts for use in synthetic processes.


 Please wait while we load your content...
Please wait while we load your content...