Acid rain formation through catalytic transformation of sulfur dioxide over clay dusts: remarkable promotion by a vicinal aluminium site†
Abstract
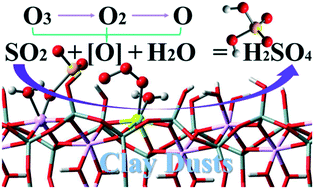
Catalytic SO2 transformation over clay dusts is crucial for acid rain formation. Clay edges trigger chemisorption of ozone, which reacts directly with SO2 instead of decomposing. A vicinal Al3+ site remarkably promotes adsorption and transformation, efficiently utilizing all O atoms in ozone (especially molecular oxygen) and producing three equivalents of H2SO4.


 Please wait while we load your content...
Please wait while we load your content...