Reductase-catalyzed tetrahydrobiopterin regeneration alleviates the anti-competitive inhibition of tyrosine hydroxylation by 7,8-dihydrobiopterin†
Abstract
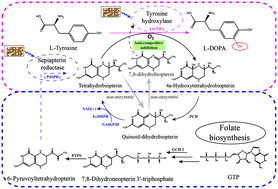
L-Tyrosine hydroxylation by tyrosine hydroxylase is a significant reaction for preparing many nutraceutical and pharmaceutical chemicals. Two major challenges in constructing these pathways in bacteria are the improvement of hydroxylase catalytic efficiency and the production of cofactor tetrahydrobiopterin (BH4). In this study, we analyzed the evolutionary relationships and conserved protein sequences between tyrosine hydroxylases from different species by PhyML and MAFFT. Finally, we selected 7 tyrosine hydroxylases and 6 sepiapterin reductases. Subsequently, the function of different groups was identified by a combined whole-cell catalyst, and a series of novel tyrosine hydroxylase/sepiapterin reductase (TH/SPR) synthesis systems were screened including tyrosine hydroxylase (from Streptosporangium roseum DSM 43021 and Thermomonospora curvata DSM 43183) and sepiapterin reductase (from Photobacterium damselae, Chlorobaculum thiosulfatiphilum and Xenorhabdus poinarii), namely as SrTH/PdSPR, SrTH/CtSPR, SrTH/XpSPR and TcTH/PdSPR, which can synthesize L-Dopa by hydroxylating L-tyrosine in Bacillus licheniformis. Furthermore, we analyzed the characterization of SrTH by enzyme catalysis and demonstrated that 7,8-dihydrobiopterin (BH2) formed by BH4 autooxidation was an anticompetitive inhibitor on SrTH. Finally, pure dihydropteridine reductase from Escherichia coli (EcDHPR) was added to the solution, and L-Dopa could be continually synthesized after 3 h, which was improved by 86% at 6 h in the catalytic reaction by SrTH. This indicates that BH4 regeneration can alleviate the inhibition by BH2 during tyrosine hydroxylation. This study provides a good starting point and theoretical foundation for further modification to improve the catalytic efficiency of tyrosine hydroxylation by tyrosine hydroxylase.


 Please wait while we load your content...
Please wait while we load your content...