Hydroxyapatite supported molybdenum oxide catalyst for selective oxidation of methanol to formaldehyde: studies of industrial sized catalyst pellets†
Abstract
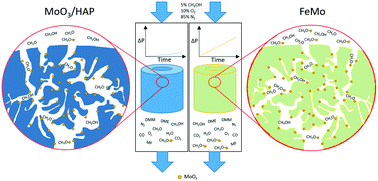
In this study, 10 wt% MoO3 supported on hydroxyapatite (HAP, Ca5(OH)(PO4)3) pellets were investigated as catalysts for the selective oxidation of methanol to formaldehyde. HAP was synthesized by co-precipitation and pressed into industrial sized pellets with different densities (1.18–1.76 g cm−3) to investigate the influence of pellet density and porosity on the catalytic performance. The pellets were wet impregnated to obtain 10 wt% MoO3. The catalysts were characterized by X-ray diffraction (XRD), N2 physisorption, scanning electron microscopy coupled with energy dispersive X-ray spectroscopy (SEM–EDX), inductively coupled plasma optical emission spectroscopy (ICP-OES) and Hg-porosimetry. The catalytic performance was evaluated at 250–400 °C for up to 100 h continuous operation in a single pellet reactor with 300 NmL min−1, 5 vol% MeOH, and 10 vol% O2 in N2. The MoO3/HAP pellets lost between 10% and 23% of their initial activity while an industrial MoO3/Fe2(MoO4)3 reference catalyst lost ∼11% within 100 h on stream at 350 °C. Despite the MoO3/HAP catalysts not outperforming the FeMo reference, it was still deemed of interest for further study and optimization as the difference was relatively small. Especially since the pellets weight loss due to Mo volatilization was >70% lower compared with the FeMo catalysts within a 118.5 h test. A random pore model, together with overall effectiveness factor calculations for both powder and pellets, was used to calculate the pellet activity from measured powder activity, providing reasonable agreement between the calculated and the observed rate constants for the pellets. An increase in both activity and selectivity to formaldehyde was obtained with decreasing pellet density, due to decreasing pore diffusion limitations of both methanol into and formaldehyde out of the pellet. At 350 °C the lightest (and weakest) pellet achieved an initial selectivity to formaldehyde of 87.5% at 25.1% conversion. The primary by-products were dimethoxymethane (DMM) (C based selectivity of 6%) and CO (C based selectivity of 4.7%). The catalysts are promising for application at the front-end of an industrial formaldehyde synthesis reactor to limit the loss of MoO3 due to evaporation with accompanied increase in reactor pressure drop.


 Please wait while we load your content...
Please wait while we load your content...