Insight into the chemoselective aromatic vs. side-chain hydroxylation of alkylaromatics with H2O2 catalyzed by a non-heme imine-based iron complex†
Abstract
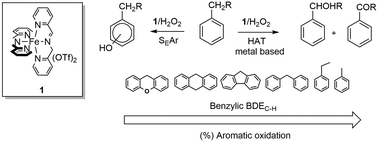
The oxidation of a series of alkylaromatic compounds with H2O2 catalyzed by an imine-based non-heme iron complex prepared in situ by reaction of 2-picolylaldehyde, 2-picolylamine, and Fe(OTf)2 in a 2 : 2 : 1 ratio leads to a marked chemoselectivity for aromatic ring hydroxylation over side-chain oxidation. This selectivity is herein investigated in detail. Side-chain/ring oxygenated product ratio was found to increase upon decreasing the bond dissociation energy (BDE) of the benzylic C–H bond in line with expectation. Evidence for competitive reactions leading either to aromatic hydroxylation via electrophilic aromatic substitution or side-chain oxidation via benzylic hydrogen atom abstraction, promoted by a metal-based oxidant, has been provided by kinetic isotope effect analysis.


 Please wait while we load your content...
Please wait while we load your content...