Catalytic conversion of alkynes to α-vinyl sulfides mediated by carbene-linker-carbene (CXC) rhodium and iridium complexes†
Abstract
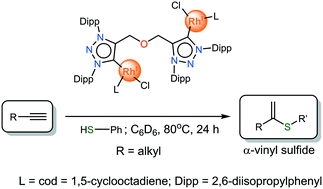
The catalytic activity of a set of mono- and bimetallic Rh(I) and Ir(I) complexes bearing carbene-linker-carbene (CXC) bis-triazolylidene ligands (with X = O, N) coordinated in a bridging or chelating fashion was evaluated in the hydrothiolation of alkynes. The hydrothiolation of 1-hexyne with thiophenol in the absence of an external base or other additives was selected as a model reaction. All rhodium complexes are highly selective catalysts towards Markovnikov product formation and display superior activity compared to the related iridium derivatives. DFT calculations were carried out to rationalize the reaction mechanism and selectivity of this process. Neutral dinuclear [Rh2Cl2(cod)2(μ-COC)] was found to be the most effective catalyst for this transformation. Its applicability was further studied towards the hydrothiolation of different alkyl and aryl alkynes using predominantly aryl thiols and proved to be one of the most active and selective catalysts towards the α-vinyl sulfide product to date.


 Please wait while we load your content...
Please wait while we load your content...