Enantioselective “organocatalysis in disguise” by the ligand sphere of chiral metal-templated complexes
Abstract
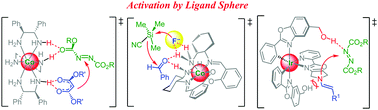
Asymmetric catalysis holds a prominent position among the important developments in chemistry during the 20th century. This was acknowledged by the 2001 Nobel Prize in chemistry awarded to Knowles, Noyori, and Sharpless for their development of chiral metal catalysts for organic transformations. The key feature of the catalysts was the crucial role of the chiral ligand and the nature of the metal ions, which promoted the catalytic conversions of the substrates via direct coordination. Subsequently the development of asymmetric organic catalysis opened new avenues to the synthesis of enantiopure compounds, avoiding any use of metal ions. Recently, an alternative approach to asymmetric catalysis emerged that relied on the catalytic functions of the ligands themselves boosted by coordination to metal ions. In other words, in these hybrid chiral catalysts the substrates are activated not by the metal ions but by the ligands. The activation and enantioselective control occurred via well-orchestrated and custom-tailored non-covalent interactions of the substrates with the ligand sphere of chiral metal complexes. In these metal-templated catalysts, the metal served either as a template (a purely structural role), or it constituted the exclusive source of chirality (metal-centred chirality due to the spatial arrangement of achiral or chiral bi-/tridentate ligands around an octahedral metal centre), and/or it increased the Brønsted acidity of the ligands. Although the field is still in its infancy, it represents an inspiring combination of both metal and organic catalysis and holds major unexplored potential to push the frontiers of asymmetric catalysis. Here we present an overview of this emerging field discussing the principles, applications and perspectives on the catalytic use of chiral metal complexes that operate as “organocatalysts in disguise”. It has been demonstrated that these chiral metal complexes are efficient and provide high stereoselective control in asymmetric hydrogen bonding catalysis, phase-transfer catalysis, Brønsted acid/base catalysis, enamine catalysis, nucleophilic catalysis, and photocatalysis as well as bifunctional catalysis. Also, many of the catalysts have been identified as highly effective catalysts at remarkably low catalyst loadings. These hybrid systems offer many opportunities in the synthesis of chiral compounds and represent promising alternatives to metal-based and organocatalytic asymmetric transformations.


 Please wait while we load your content...
Please wait while we load your content...