Visible light photoredox-catalysed remote C–H functionalisation enabled by 1,5-hydrogen atom transfer (1,5-HAT)
Abstract
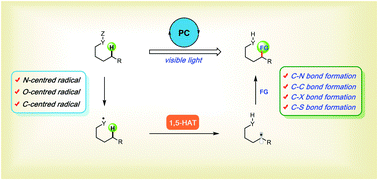
The generation of heteroatom-centred radicals (X˙), followed by intramolecular 1,5-hydrogen atom transfer (1,5-HAT) and the functionalisation of the translocated carbon-centred radicals, is the basic mechanism of the classic Hofmann–Löffler–Freytag (HLF) reaction and the Barton reaction. The chemoselectivity of the 1,5-HAT process is different from that of the transition metal-catalysed counterpart, providing, therefore, a complementary tool for remote C(sp3)–H bond functionalisation. There is a recent resurgence in this research field due to the emergence of visible light photocatalysis. This tutorial review summarises the recent progress in the remote functionalisation of C–H bonds featuring a key 1,5-HAT step with particular focus on photoredox-catalyzed remote C–H functionalisation.


 Please wait while we load your content...
Please wait while we load your content...