Spectroelectrochemical study of the reduction of 2-methyl-9H-thioxanthene-9-one and its S,S-dioxide and electronic absorption spectra of their molecular ions†
Abstract
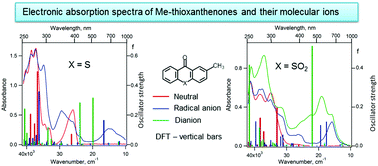
2-Methyl-9H-thioxanthene-9-one (1) and its S,S-dioxide (2) are the precursors of pendant groups that determine the reduction potentials of electro-active polyimides, which exhibit electrochromic behavior and are used in organic electronics. Electrochemical reduction of 1 and 2 leads to the formation of the corresponding persistent radical anions and dianion (for S,S-dioxide). Using 3D spectroelectrochemistry, all anions have been shown to exhibit strong absorption in the UV-VIS-NIR wavelength region. Electronic absorption spectra of 1 and 2 and their negative ions were interpreted using time-dependent DFT. According to the calculations, the most intense electronic transitions of the dianions 12− and 22− in the visible region exhibit hypsochromic shift compared to the intense transitions of the corresponding radical anions and have much higher oscillator strengths, which was confirmed experimentally for 2. An empirical kinetic model was proposed based on the analysis of the total charge passed through the cell during electrolysis and on the established mechanism of electrochemical reduction. This model perfectly described the UV-VIS-NIR optical density time dependences observed on 3D spectroelectrochemical surfaces for both compounds 1 and 2. This made it possible to explain the differences in the electrochromic behaviour of ambibolar electro-active polyimides with pendant groups based on 1, 2.


 Please wait while we load your content...
Please wait while we load your content...