Excited-state engineering of oligothiophenes via phosphorus chemistry towards strong fluorescent materials†
Abstract
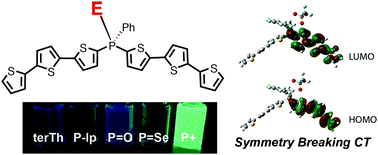
Due to efficient intersystem crossing (ISC), combined with efficient non-radiative processes of the triplet excited state, oligothiophenes generally exhibit very weak photoluminescence. Phosphorus (P)-bridged terthiophenes (P-terThs) and phosphorus (P)-bridged bithiophenes (P-biThs) were synthesized. The diverse and well-defined P-chemistry has been applied to fine tune the photophysical properties of these materials. The asymmetric electronic coupling between the P-center and terThs suppressed the electronic interactions of two terTh and biTh moieties in the ground state S0. Particularly, P-terThs and P-biThs having a positively charged P(+)-center induce pronounced asymmetric electronic environments on the two terThs and two biThs, respectively, which allows relaxation from the initial excited state via symmetry breaking charge transfer (SBCT) to give the charge separated state SSBCT. P-terThs and P-biThs having a positively charged P(+)-center exhibit stronger SBCT than others, which may result in a weaker ISC of oligothiophenes, and consequently lead to the photoluminescence quantum yields (PLQYs) being as high as 71% and 39%, respectively. The current study uncovered detailed insights on the effects of phosphorus chemistry on the SBCT of oligothiophenes and their resulting effects on the photophysical properties of P-bridged oligothiophenes, which have not been previously addressed in oligothiophenes.


 Please wait while we load your content...
Please wait while we load your content...