Conversion of carbon dioxide to a novel molecule NCNBO− mediated by NbBN2− anions at room temperature†
Abstract
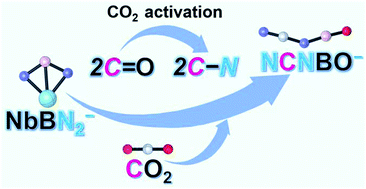
The activation of carbon dioxide (CO2) mediated by NbBN2− cluster anions under the conditions of thermal collision has been investigated by time-of-flight mass spectrometry combined with density functional theory calculations. Two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds in the CO2 molecule are completely broken and two C–N bonds are further generated to form the novel molecule NCNBO−. To the best of our knowledge, this new molecule is synthesized and reported for the first time. In addition, one oxygen atom transfer channel produces another product, NbBN2O−. Both of the Nb and B atoms in NbBN2− donate electrons to reduce CO2, and the carbon atom originating from CO2 serves as an electron reservoir. The reaction of NbB− with N2 was also investigated theoretically, and the formation of NbBN2− from this reaction is thermodynamically and kinetically quite favorable, indicating that NCNBO− might be produced from the coupling of N2 and CO2 mediated by NbB− anions.
O double bonds in the CO2 molecule are completely broken and two C–N bonds are further generated to form the novel molecule NCNBO−. To the best of our knowledge, this new molecule is synthesized and reported for the first time. In addition, one oxygen atom transfer channel produces another product, NbBN2O−. Both of the Nb and B atoms in NbBN2− donate electrons to reduce CO2, and the carbon atom originating from CO2 serves as an electron reservoir. The reaction of NbB− with N2 was also investigated theoretically, and the formation of NbBN2− from this reaction is thermodynamically and kinetically quite favorable, indicating that NCNBO− might be produced from the coupling of N2 and CO2 mediated by NbB− anions.


 Please wait while we load your content...
Please wait while we load your content...