Hydration and counterion binding of aqueous acetylcholine chloride and carbamoylcholine chloride†
Abstract
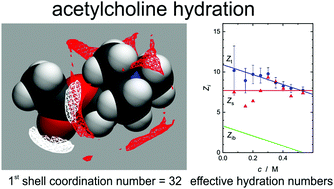
The hydration and Cl− ion binding of the neurot†ransmitter acetylcholine (ACh+) and its synthetic analogue, carbamoylcholine (CCh+), were studied by combining dilute-solution conductivity measurements with dielectric relaxation spectroscopy and statistical mechanics calculations at 1D-RISM and 3D-RISM level. Chloride ion binding was found to be weak but not negligible. From the ∼30 water molecules coordinating ACh and CCh+ only ∼1/3 is affected in its rotational dynamics by the cation, with the majority – situated close to the hydrophobic moieties – only retarded by a factor of ∼2.5. At vanishing solute concentration cations and the ∼3–4 H2O molecules hydrogen bonding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the solute exhibit similar rotational dynamics but increasing concentration and temperature markedly dehydrates ACh+ and CCh+.
O group of the solute exhibit similar rotational dynamics but increasing concentration and temperature markedly dehydrates ACh+ and CCh+.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...