Assessing nickel oxide electrocatalysts incorporating diamines and having improved oxygen evolution activity using operando UV/visible and X-ray absorption spectroscopy†
Abstract
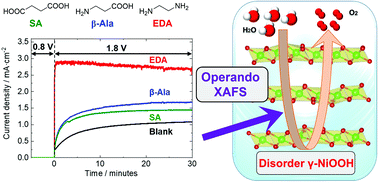
The electrolysis of water using renewable energy is a promising approach to developing a sustainable hydrogen-based economy. To improve the efficiency of this process, it will be necessary to develop highly active electrocatalysts that promote the oxygen evolution reaction (OER). In the present study, the OER activity of a nickel oxide electrocatalyst was dramatically improved following the addition of a diamine to the electrolyte solution during electrodeposition. Operando UV/vis absorption spectroscopy was used to assess a number of nickel catalysts containing various diamines and other organic compounds. The data indicate that Ni(II) complexes were formed with the diamines during electrodeposition. Consequently, the catalytic activity of these materials was enhanced based on increased concentrations of active reaction sites for the OER process. Ni K-edge X-ray absorption spectra showed that these catalysts were composed of γ-NiOOH with a Ni3.6+ valence state. The coordination of the diamine molecules to the γ-NiOOH produced structural distortion that contributed to improved OER activity. This structural distortion is likely the most important factor in enhancing the OER activity of inorganic–organic composite catalysts.


 Please wait while we load your content...
Please wait while we load your content...