Microelectrode-based transient amperometry of O2 adsorption and desorption on a SrTiO3 photocatalyst excited under water†
Abstract
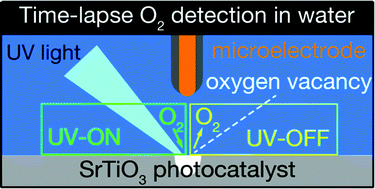
Oxygen evolution at water–solid interfaces is a key reaction for sustainable energy production. Although some intermediate states have been detected in transient absorption spectroscopy, the O2 evolution kinetics after the multi-step, four-electron oxidation of water remain unknown. In this study, transient amperometry with a microelectrode was applied to operando O2 detection over Al-doped SrTiO3 particles doubly loaded with RhCrOx and CoOy cocatalysts, an efficient photocatalyst for the overall water-splitting reaction. Electrochemical O2 detection at intervals of 0.1 s unexpectedly indicated instantaneous O2 adsorption and desorption in addition to steady, photocatalytic O2 evolution on the photocatalyst modified under intense light irradiation. We hypothesized that electrons excited in the conduction band were transferred to O2 in water thorough Ti cations neighboring an oxygen anion vacancy on the modified Al-doped SrTiO3. The negatively charged O2 was then bound to the Ti cations. It was neutralized and released when shaded through electron back-transfer to the conduction band. The hypothesized mechanism for O2 adsorption and desorption was compared with the photoinduced O2 desorption known to occur on anion vacancies of TiO2(110). The microelectrode-based transient amperometry demonstrated in this paper will be applied to many other phenomena at liquid–solid interfaces.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...