Transient absorption spectroscopy of the electron transfer step in the photochemically activated polymerizations of N-ethylcarbazole and 9-phenylcarbazole†
Abstract
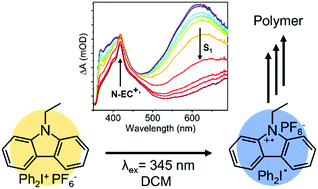
The polymerization of photoexcited N-ethylcarbazole (N-EC) in the presence of an electron acceptor begins with an electron transfer (ET) step to generate a radical cation of N-EC (N-EC˙+). Here, the production of N-EC˙+ is studied on picosecond to nanosecond timescales after N-EC photoexcitation at a wavelength λex = 345 nm using transient electronic and vibrational absorption spectroscopy. The kinetics and mechanisms of ET to diphenyliodonium hexafluorophosphate (Ph2I+PF6−) or para-alkylated variants are examined in dichloromethane (DCM) and acetonitrile (ACN) solutions. The generation of N-EC˙+ is well described by a diffusional kinetic model based on Smoluchowski theory: with Ph2I+PF6−, the derived bimolecular rate coefficient for ET is kET = (1.8 ± 0.5) × 1010 M−1 s−1 in DCM, which is consistent with diffusion-limited kinetics. This ET occurs from the first excited singlet (S1) state of N-EC, in competition with intersystem crossing to populate the triplet (T1) state, from which ET may also arise. A faster component of the ET reaction suggests pre-formation of a ground-state complex between N-EC and the electron acceptor. In ACN, the contribution from pre-reaction complexes is smaller, and the derived ET rate coefficient is kET = (1.0 ± 0.3) × 1010 M−1 s−1. Corresponding measurements for solutions of photoexcited 9-phenylcarbazole (9-PC) and Ph2I+PF6− give kET = (5 ± 1) × 109 M−1 s−1 in DCM. Structural modifications of the electron acceptor to increase its steric bulk reduce the magnitude of kET: methyl and t-butyl additions to the para positions of the phenyl rings (para Me2Ph2I+PF6− and t-butyl-Ph2I+PF6−) respectively give kET = (1.2 ± 0.3) × 1010 M−1 s−1 and kET = (5.4 ± 1.5) × 109 M−1 s−1 for reaction with photoexcited N-EC in DCM. These reductions in kET are attributed to slower rates of diffusion or to steric constraints in the ET reaction.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...